ANA at the Vaccination Forefront
Read how ANA has been at the forefront of COVID-19 vaccine advocacy, and find other COVID-19 vaccination related resources.

ANA Advocacy and Legislative Efforts
ANA’s Department of Policy and Government Affairs continues to work with Congress and the administration on issues of importance surrounding COVID-19. Learn about the latest efforts, find valuable federal and state resources, and discover how nurses and nurse advocates can get involved.
Click Here for more information on ANA's legislative and regulatory advocacy related to Covid-19.
ANA Surveys and Resources
- See the results from the Pulse on the Nation’s Nurses COVID-19 Survey Series
- Click here for a downloadable pdf of COVID-19 Vaccine Resource Links for Nurses
- Find general immunization resources on the ANA Immunize webpage
ANA in the News
ANA President Signs on to COVID-19 Vaccination Safety Statement
Following a Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) meeting June 22nd 2021, leaders of several health care organizations, including ANA President Ernest Grant, signed on to a statement affirming the safety and effectiveness of COVID-19 vaccines. In the meeting, ACIP members discussed the latest data on reports of mild cases of myocarditis and pericarditis following COVID-19 vaccination among younger people. The statement read, in part, “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.” They concluded that “the vaccines are safe and effective, and they prevent COVID-19 illness. They will help protect you and your family and keep your community safe. We strongly encourage everyone age 12 and older who are eligible to receive the vaccine under Emergency Use Authorization to get vaccinated, as the benefits of vaccination far outweigh any harm.”
ANA Joins the COVID-19 Community Corps
On April 1, 2021, ANA became a founding member of the COVID-19 Community Corps, a U.S. Department of Health and Human Services (HHS) initiative to increase vaccine confidence while reinforcing basic prevention measures. Join the COVID-19 Community Corps here.
ANA President Grant Participates in Phase III Covid-19 Vaccine Clinical Trial
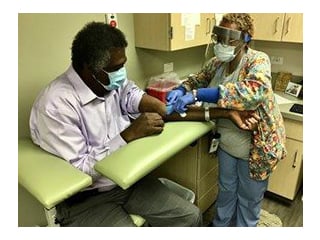
In the fall of 2020, then ANA President Dr. Ernest Grant voluntarily participated in the Moderna clinical trial to raise vaccine awareness and support the nursing profession.
"I wanted to stand in solidarity with my colleagues to alleviate any concerns and issues they may have, to see that their national leader has put his life on the line, who didn't know at the time how effective the vaccines would be, who didn't know if he would get the vaccine or a placebo," Grant said in an interview with Healthline. "I wanted to step up and do my part."
During an appearance on Good Morning America, Grant shared that he recognized the need for more people of color to participate in clinical trials. This was one of the driving factors in his decision to join the Moderna trial.
He has urged individuals, who are wary of the vaccine, to talk with someone they trust. Grant also encourages Black, LatinX, and all nurses of color to connect with their patients to share culturally relevant information and answer questions about getting vaccinated against COVID-19. This is a simple and effective way to serve as role models and encourage people of color to get safely vaccinated. This can also help to decrease disproportionate COVID-19 infection rates in communities of color.
After receiving his second shot, Grant experienced some of the expected, mild side effects including fatigue and chills. However, he has not reported any side effects since. Through an app on his phone, Grant recorded how he was feeling twice a day for two weeks. Clinical trial staff members also called him in the third and fourth week to check on him.
Grant's trial has since been unblinded and he learned that he did receive the vaccine, rather than the placebo. He has elected to remain in the study for the full two years, as health care professionals continue to gather data on the on the effects of the vaccine and the length of anticipated immunity.